Harnessing the Power of the Innate Immune System: Introducing Ceragenins, a Versatile Immunomodulatory drug platform for Eradicating Pseudomonas Aeruginosa and a Range of Bacterial and Fungal Infections in Cystic Fibrosis and other diseases.
Overview
At Kinnear Pharmaceuticals, we’re pioneering the future of anti-infective and anti-inflammatory therapies. As a dynamic, preclinical stage company, our focus is on developing innovative Ceragenin-based treatments to address diseases that currently lack effective solutions. Our mission is deeply rooted in discovering, developing, and bringing to market revolutionary product candidates.
Kinnear Pharmaceuticals is a subsidiary of N8 Biosciences (www.n8medical.com), a holding company dedicated to the broad application of Ceragenins. N8 Biosciences focus includes the commercialization of CeraShield technology, a specialized coating for medical devices aimed at preventing hospital-acquired infections (HAIs).
Science
Ceragenins : A Synthesis of Nature and Innovation

Inspired by nature
Unique capabilities

Proven and pat
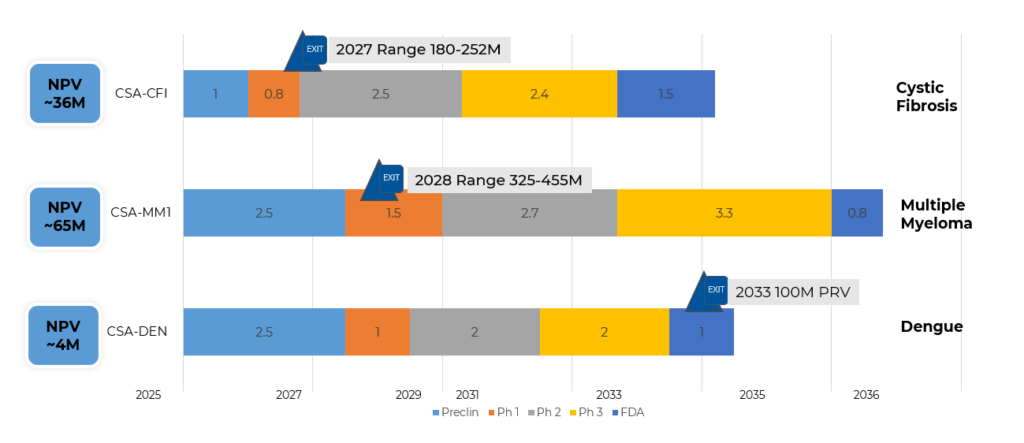
Pipeline
Our Key Focus: Developing a Versatile Antibiotic to Prevent and Treat Cystic Fibrosis, Multiple Myeloma, and Dengue.
News
FDA grants QIDP Designation For Kinnear’s CSA-131 drug for Life-threatening Pseudomonas Bacterial Infections in CF
FDA QiDP designation is a significant milestone PARK CITY, UTAH, UNITED STATES, July 31, 2023/EINPresswire.com/ — Kinnear Pharmaceuticals, LLC, a subsidiary of
New Publication regarding Ceragenin CSA-131’s Potent Efficacy against fungal pathogen Candida auris, a New Serious Global Health Threat
Colistin Resistance on the Rise
Colistin is the last agent used to combat bacteria that are resistant to the strongest antibiotics. Colistin has remained the best tool
Lets Get in Touch!
- 1 (702) 285-5740
- info@kinnearpharma.com
- Fax: (877) 686-3318
- 3960 Howard Hughes Parkway, Las Vegas, NV, 89169
Follow now
